Two different parameters can be used to describe the energy content of substances. Particularly in applications in the hydrogen sector, the question often arises: Does the low (net) or high (gross) heating value have to be used to calculate the energy content of hydrogen?
General Info
The lower heating value describes the energy content of a substance that can be utilized as heat through simple combustion. With such „simple“ combustion, however, some energy is generally always lost in the hot flue gas. If this energy contained in the flue gas is also utilized, this is referred to as the higher heating value.
The low or high heating value can be related either to a volume (for gases usually in kWh/m3) or to a weight (kWh/kg). Find out more here: Energieinhaltsrechner
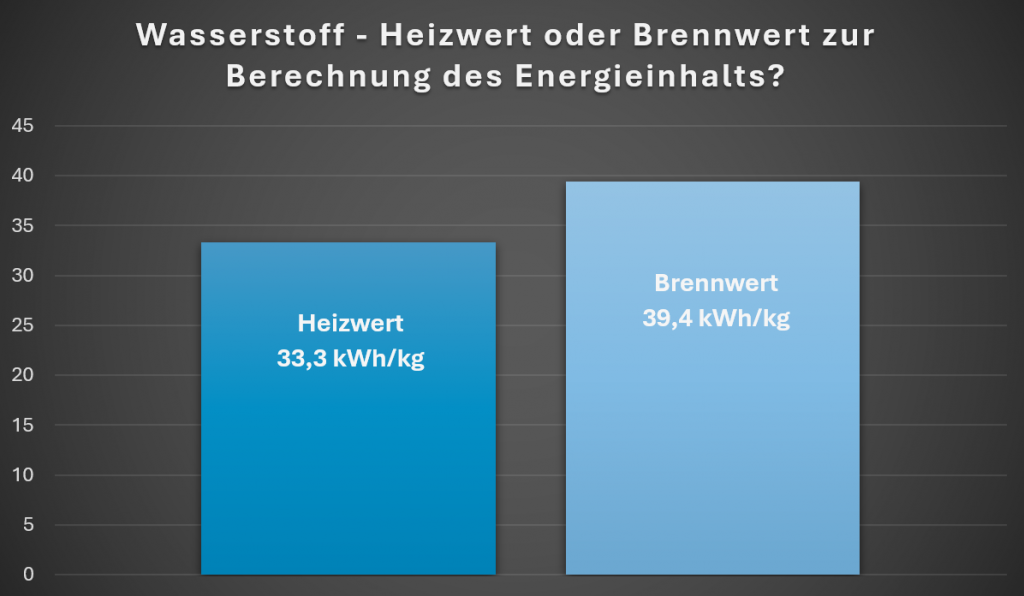
However, hydrogen is rarely „burned“ directly, but is usually utilized in an electrochemical process. So which energy content figure should be used for calculations in the hydrogen sector? Higher heating value (39.4kWh/kg) or lower heating value (33.3kWh/kg)?
Background
The underlying process is decisive for the answer: the combustion of hydrogen-containing compounds produces water, which can be liquid or gaseous depending on the type of process. If the water escapes unused as water vapor, the lower heating value is used for the calculation. However, if it liquefies within the process under consideration, the enthalpy of vaporization of the water is also „used“, which is why the higher heating value must be applied here. The discrepancy between the two values therefore corresponds to the enthalpy of vaporization of the product water.
Depending on the type and utilization of a fuel cell, the „exhaust gas“ (water/water vapour) is produced in different compositions. In most H2 cars, for example, a PEM fuel cell is installed, which produces mainly water vapor, but sometimes also liquid water. However, the liquid water is usually only produced at a later stage through condensation and not directly in the electrochemical process, which is why the enthalpy of vaporization cannot be used during power generation, at most the waste heat can be harvested.
Conclusion
In order to calculate the energy content that can ultimately be converted into electricity within the fuel cell, the lower heating value should therefore be used in the vast majority of cases!
If, for example, a process is considered in which the resulting (condensation) heat is also utilized, the higher heating value can also be used to calculate the usable energy, depending on the design of the fuel cell. For special fuel cell types, it cannot generally be ruled out that the enthalpy of vaporization cannot also be utilized electrochemically. However, the use of a suitable efficiency (which in the best case also describes the percentage share of thermal energy and electrical energy) is more important for calculating the energy yield anyway, as the possible fluctuations here are usually far greater than the percentage difference between the lower and higher heating value.
More info concerning enrgy contents: https://www.energie-lexikon.info/wasserstoff.html